Sapphire Properties
Sapphire crystal, with a chemical composition of aluminum oxide and a chemical formula (α-Al2O3), is formed by the combination of three oxygen atoms and two aluminum atoms in a covalent bond type, with a hexagonal crystal structure. Sapphire single crystal is a simple coordination type oxide crystal, exhibiting anisotropy, and belongs to the hexagonal crystal system.

Lattice parameters: a=b=4.758 Å (0.4758 nm), c=12.991 Å (1.2991 nm), α=β=90°, γ=120°. Refractive index: 1.762-1.770. Birefringence: 0.008~0.010.
Sapphire Chemical properties
Sapphire crystal has extremely stable chemical properties, generally insoluble in water and not corroded by acids or alkalis, only corroded by hydrofluoric acid, phosphoric acid, and molten potassium hydroxide at high temperatures (300°C).
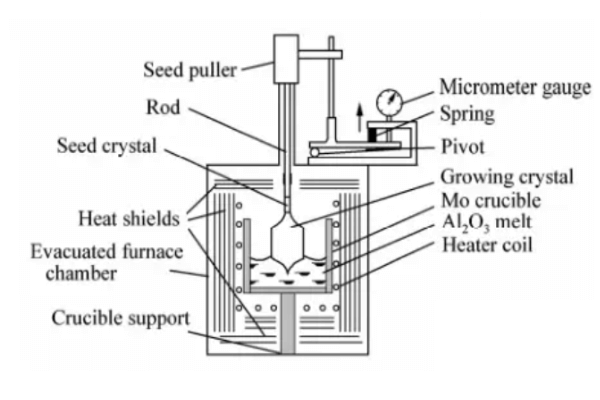
Kyropoulos method
Sapphire Physical properties
Sapphire crystal has a high hardness, with a Mohs hardness of 9, second only to the hardest diamond. It has excellent transparency, thermal conductivity, and electrical insulation properties, good mechanical properties, and features of wear resistance and erosion resistance. The sapphire melting point is 2050°C, and the boiling point is 3500°C. The highest working temperature can reach 1900°C. Sapphire glass has excellent thermal properties, excellent electrical properties and dielectric properties, and is resistant to chemical corrosion. It is resistant to high temperatures, has good thermal conductivity, high hardness, transmits infrared light, and has good chemical stability.
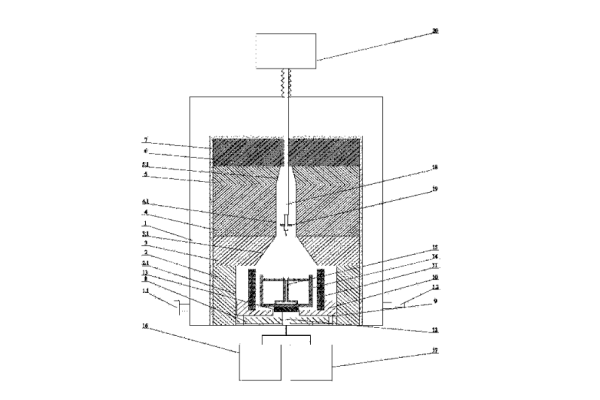
Edge-Defined Film-Fed Growth Method
As an important technical crystal, sapphire crystal has been widely used in various fields of science and technology, national defense, civilian industry, and electronics technology.
Sapphire Growth Methods
There are two main methods for the synthesis of artificial sapphire crystals: one is the long crystal process originated from the former Soviet Union: the Czochralski method; the other is the long crystal process originated from the United States: the hydrothermal exchange method.
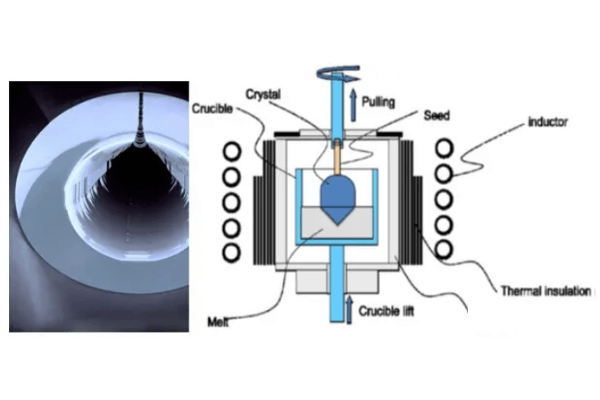
Czochralski Method
| Chamical Formula | Sapphire (Al2O3) |
|---|---|
| Crystal Structure | Hexagonal |
| Density | 3.98 ~ 4.1 g/cm^3 |
| Melting Point | 2040 °C |
| Hardness (Mohs) | 9 |
| Young`s Modulus /GPa | 380 |
| Tensile Strength/Mpa | 400 |
| Thermal Conductivity | 24 W / (m K) |
| Temperature dependence of refractive index | 8.8 x 10^-6 K^-1 |
| Absorption Coefficient | 0.5 ~ 6.0 cm-1 |
| Index of refraction | 1.769 (parallel to C-axis) |
| Index of refraction | 1.760 (perpendicular to C-axis) |
| Infrared of penetrable index | >85% |